Despite these extraordinary successes, vaccines and their constituents (e.g., the mercury compound thimerosal , formerly used as a preservative) have come under attack in some countries as causes of neurodevelopmental disorders, such as autism and attention-deficit hyperactivity disorder; diabetes; and a variety of allergic and autoimmune diseases. Although millions of lives are saved by vaccines each year and countless cases of postinfection disability are averted, some segments of the public are increasingly unwilling to accept any risk whatsoever of vaccine-associated complications (severe or otherwise), and resistance to vaccination is growing.
No medical procedure is absolutely risk-free, and the risk to the individual must always be balanced with benefits to the individual and to the population must always be balanced with benefits to the individual and to the population at large. This dichotomy poses two essential challenges for the medical and public health communities with respect to vaccines: (1) to create more effective and ever-safer vaccines, and (2) to educate patients and the general public more fully about the benefits as well as the risks of vaccine use. Because immunity to infectious diseases is acquired only by infection itself or by immunization, sustained vaccination programs for each birth cohort will continue to be necessary to control vaccine-preventable infectious diseases until and unless their etiologic agents can be eradicated from every region of the world
 An unwavering scientific and public health commitment to immunization is essential in countering public distrust and political pressure to legislate well-intentioned but ill-informed vaccine safety laws in response to the concerns of organized antivaccine advocacy groups. Ironically, it is the public health success of vaccines that has created a significant part of the problem: because the major fatal and disabling diseases of childhood are only rarely seen today in the United States, parents and young practitioners most likely will never have seen tetanus, diphtheria, Haemophilus influenzae disease, polio, or measles. Under these circumstances, the risks of immunization can easily (if erroneously) be perceived to outweigh the benefits, and this perception can be fueled by inaccurate information, poor science , and zealous advocacy. Caregivers must be prepared to educate parents about the importance of childhood immunization and to address their concerns effectively.
An unwavering scientific and public health commitment to immunization is essential in countering public distrust and political pressure to legislate well-intentioned but ill-informed vaccine safety laws in response to the concerns of organized antivaccine advocacy groups. Ironically, it is the public health success of vaccines that has created a significant part of the problem: because the major fatal and disabling diseases of childhood are only rarely seen today in the United States, parents and young practitioners most likely will never have seen tetanus, diphtheria, Haemophilus influenzae disease, polio, or measles. Under these circumstances, the risks of immunization can easily (if erroneously) be perceived to outweigh the benefits, and this perception can be fueled by inaccurate information, poor science , and zealous advocacy. Caregivers must be prepared to educate parents about the importance of childhood immunization and to address their concerns effectively.DEFINITIONS
The terms vaccination and immunization are often used interchangeably, although technically the former denotes the administration of a vaccine, whereas the latter refers to the induction or provision of immunity by any means, active or passive. Thus vaccination does not guarantee immunization, and immunization may not involve vaccine.
PRINCIPLES OF IMMUNIZATION
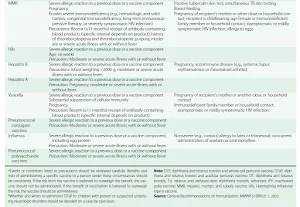
The immune system, composed of a variety of cell types and soluble factors, is geared toward the recognition of and response to "foreign" substances termed antigens. Vaccines convey antigens from living or killed microorganisms (or protein or carbohydrate molecules derived from these antigens) to elicit immune responses that are generally protective but can occasionally backfire and cause harm to the recipient. Specific immune responses, which interrupt the infectious process, generally take the form of immunoglobulin proteins called antibodies and/or activated immune cells that recognize particular antigens from an infectious agent. Immunity is medically induced by active or passive immunization. Active immunization-i.e., the administration of a vaccine-induces immunity that is usually long-lasting and is sometimes life-long. In contrast, passive immunization-i.e., the administration of exogenously produced immune substances or of protective products made in animals-elicits temporary immunity that dissipates with the turnover of the administered protective substances. Used together, the two methods can produce a complementary effect; this is the case, for example, with the coadministration of hepatitis B vaccine and hepatitis B immune globulin. Caution is required, however: the combination of active and passive immunization can also interfere with the development of immunity-e.g., when measles vaccine is administered within 6 weeks of measles immunoglobulin.
When multiple species or serotypes of an organism exist and share common, cross-reactive antigens, vaccination may induce broad immunity to all or most of the related forms or may result in serotype-specific immunity against the immunizing strain alone. One of the virtues of whole-organism vaccines is their potential to contain all the possibility of adverse responses to reactive but nonprotective antigens present in the mix. Because the immune response is genetically controlled, all individuals cannot be expected to respond identically to the same vaccine. Additional vaccine constituents affect immunogenicity, efficacy, and safety and may render one formulation superior to another formulation of the same antigens.
Approaches to Active Immunization The two standard approaches to active immunization are the use of live, generally attenuated infectious agents (e.g., measles virus); and the use of inactivated agents (e.g., influenza virus), their constituents (e.g., Bordetella pertusis), or their products , which are now commonly obtainable through genetic engineering (e.g., hepatitis B vaccine). For many diseases (e.g., poliomyelitis), both live and inactivated vaccines have been employed, each offering advantages and disadvantages.
Live attenuated vaccines consisting of selected or genetically altered organisms that are avirulent or dramatically attenuated, yet remain immunogenic, typically generate long-lasting immunity. These vaccines are designed to cause a subclinical or mild illness and an immune response that mimics natural infection. They offer the advantage of microbial replication in vivo, which simulates natural infection; they may confer life-long protection with one dose; they can present all potential antigens, including those made only in vivo, thus overcoming immunogenetic restrictions in some hosts; and they can reach the local sites most relevant to the induction of protective immunity.
 Nonliving vaccines typically require multiple doses and periodic boosters for the maintenance of immunity. The exception cannot be boosted by additional exposures because polysaccharides do not elicit immunologic memory. Nonliving vaccines administered parenterally fail to induce mucosal immunity because they lack a delivery system that can effectively transport them to local mucosal antigen-processing cells. Nonetheless, nonliving parenteral vaccines can be extremely in nearly 100% of recipients. Currently available nonliving vaccines consist of inactivated whole organisms (e.g., plague vaccine), detoxified protein exotoxins (e.g., tetanus toxoid), recombinant protein antigens (e.g., hepatitis B vaccine), or carbohydrate antigens-either soluble purified capsular material (e.g., serotype-specific Streptococcus pneumoniae polysaccharides) or polysaccharide conjugated to a protein carrier to induce a memory response (e.g., Hib polysaccharide conjugated to a suitable protein moiety).
Nonliving vaccines typically require multiple doses and periodic boosters for the maintenance of immunity. The exception cannot be boosted by additional exposures because polysaccharides do not elicit immunologic memory. Nonliving vaccines administered parenterally fail to induce mucosal immunity because they lack a delivery system that can effectively transport them to local mucosal antigen-processing cells. Nonetheless, nonliving parenteral vaccines can be extremely in nearly 100% of recipients. Currently available nonliving vaccines consist of inactivated whole organisms (e.g., plague vaccine), detoxified protein exotoxins (e.g., tetanus toxoid), recombinant protein antigens (e.g., hepatitis B vaccine), or carbohydrate antigens-either soluble purified capsular material (e.g., serotype-specific Streptococcus pneumoniae polysaccharides) or polysaccharide conjugated to a protein carrier to induce a memory response (e.g., Hib polysaccharide conjugated to a suitable protein moiety). Despite their many advantages , live vaccines are not always preferable. For example , after several decades of extensive use, live oral polio vaccine (OPV) is no longer recommended in the United States because of the rare but real risk of vaccine-associated polio due to reversion to virulence. However, the WHO continues to recommend OPV for use in the developing world because of lower costs and logistical advantages.
Approaches to Passive Immunization Passive immunization is generally used to provide temporary immunity in a person exposed to an infectious disease who has not been actively immunized; this situation can arise when active immunization is unavailable (e.g., for respiratory syncytial virus) or when active immunization simply has not been implemented before exposure (e.g., for rabies). Passive immunization is used in the treatment of certain illnesses associated with toxins (e.g., diphtheria) as well as for some snake and spider bites and as a specific or nonspecific immunosuppressant [Rho(D) immune globulin and antilymphocyte globulin, respectively]. Three types of preparations can be used in passive immunization: 1. standard human immune serum globulin for IM or IV administration; 2. special immune serum globulins with a known content of antibody to specific agents (e.g., hepatitis B virus or varicella-zoster immune globulin); and 3. specific animal antisera and antitoxins.
Postexposure Immunization For certain infections, active or passive immunization soon after exposure can prevent or attenuate disease expression. Recommended postexposure immunization regimens are shown in Table 116-1.
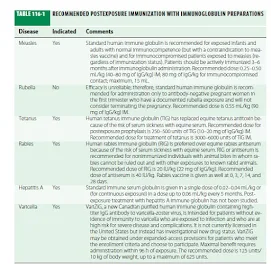 For example, giving either measles immune globulin within 6 days of exposure or measles vaccine within the first few days after exposure may prevent symptomatic infection. Nonimmune pregnant woman exposed to rubella can minimize clinical illness by postexposure passive immunization; however, this measure may fail to prevent viremia and infection of the fetus and thus may be followed by the congenital rubella syndrome. Proper immunization for tetanus plays an important role in dirty-wound management. The need for active immunization-with or without passive immunization - depends on the wound's condition and the patient's immunization history. Tetanus is rare among persons with document receipt of a primary series of tetanus toxoid doses. Tetanus immune globulin is helpful in patients with clinical tetanus , but survivors must be actively immunized since the disease does not stimulate protective levels of antitoxin antibody. Administration of rabies immune globulin plus rabies vaccine in the immediate postexposure period is highly effective in preventing disease. Similarly, for persons who have not been actively immunized, administration of hepatitis A immune globulin within 2 weeks of exposure to hepatitis A virus is likely to prevent clinical illness. Evidence also supports the efficacy of human hepatitis B immune globulin in preventing disease after exposure. While no high-titer preparation is available for postexposure protection against non-A, non-B hepatitis, standard human immune serum globulin is efficacious. VariZIG, a highly purified preparation of human antibody to varicella-zoster virus (VZV), is licensed in Canada for the prevention of varicella in nonimmune pregnant women who are exposed to infected individuals. At the time of this writing, this product is available in the United States from the Centers for Disease Control and Prevention (CDC) under an investigational new drug (IND) protocol or under an expanded-access program through the U.S. Food and Drug Administration.
For example, giving either measles immune globulin within 6 days of exposure or measles vaccine within the first few days after exposure may prevent symptomatic infection. Nonimmune pregnant woman exposed to rubella can minimize clinical illness by postexposure passive immunization; however, this measure may fail to prevent viremia and infection of the fetus and thus may be followed by the congenital rubella syndrome. Proper immunization for tetanus plays an important role in dirty-wound management. The need for active immunization-with or without passive immunization - depends on the wound's condition and the patient's immunization history. Tetanus is rare among persons with document receipt of a primary series of tetanus toxoid doses. Tetanus immune globulin is helpful in patients with clinical tetanus , but survivors must be actively immunized since the disease does not stimulate protective levels of antitoxin antibody. Administration of rabies immune globulin plus rabies vaccine in the immediate postexposure period is highly effective in preventing disease. Similarly, for persons who have not been actively immunized, administration of hepatitis A immune globulin within 2 weeks of exposure to hepatitis A virus is likely to prevent clinical illness. Evidence also supports the efficacy of human hepatitis B immune globulin in preventing disease after exposure. While no high-titer preparation is available for postexposure protection against non-A, non-B hepatitis, standard human immune serum globulin is efficacious. VariZIG, a highly purified preparation of human antibody to varicella-zoster virus (VZV), is licensed in Canada for the prevention of varicella in nonimmune pregnant women who are exposed to infected individuals. At the time of this writing, this product is available in the United States from the Centers for Disease Control and Prevention (CDC) under an investigational new drug (IND) protocol or under an expanded-access program through the U.S. Food and Drug Administration.The immune response While many constituents of infectious microorganisms and their products (e.g., exotoxins) are or can be rendered immunogenic, only some stimulate protective immune responses that can prevent infection and/or clinical illness or (as in the case of rotavirus) can attenuate illness, providing protection against severe disease but not against infection or mild illness. The immune system is complex, and many factors-including antigen composition and presentation as well as host characteristics are critical for stimulation of the desired immune responses.
The Primary Response The primary response to a vaccine antigen includes an apparent latent period of several days before immune responses can be detected. Although the immune system is rapidly activated, it takes 7-10 days for activated B lymphocytes to produce enough antibody to be detected in the circulation. The primarily IgM antibodies seen initially are rapidly produced but have only a low affinity for the antigen. After the first week, high-affinity IgG antibodies begin to be produced in quantity; this switch from IgM to IgG production requires the participation of CD4+ T-helper lymphocytes -the "middle man" of the immune response. Because precursors of T cells mature within the thymus gland, antigens that stimulate T cells are referred to as T or thymus-dependent antigens. Circulating antigen-specific T lymphocytes that implement cell-mediated immune responses are identified in the peripheral bloodstream only after several days but begin to increase immediately after antigenic stimulation.
Activation of these responses typically require co-recognition of the antigen by specific molecular species of HLA, the major histocompatibility complex, which is present on the surface of lymphocytes and macrophages. Some individuals cannot respond to one or more antigens, even when repeatedly exposed, because they do not have the genes for the particular HLA type involved in antigen recognition, processing, and presentation for an immune response. This situation is known as primary vaccine failure.
The Secondary Response Stronger and faster humoral or cell-mediated responses are elicited by a second exposure to same antigen and are detectable within days of the "booster" dose. The secondary response depends on immunologic memory induced by the primary the primary exposure and is characterized by a marked proliferation of IgG antibody-producing B lymphocytes and/or effector T cells. Pure polysaccharide antigens, such as the first-generation pneumococcal vaccine, evoke immune responses that are independent of T cells and are not enhanced by repeated administration. However, conjugation of the same polysaccharide to a suitable protein converts the carbohydrate antigen into one that is T cell-dependent and able to induce immunologic memory and secondary responses to reexposure . Although levels of vaccine-induced antibodies may decline over time, revaccination or infection generally elicits a rapid ( anamnestic) protective secondary response consisting of IgG antibodies, with little or no detectable IgM. Thus, a lack of measurable antibody in an immunized individual does not necessarily indicate secondary vaccine failure. Similarly, the mere presence of detectable antibodies after immunization does not ensure clinical protection: the level of circulating antibody may need to exceed a threshold value in order to mediate protection (e.g., 0.01 IU/mL for tetanus antitoxin).
Mucosal Immunity Some pathogens are confined to and replicate only at mucosal surfaces (e.g., Vibrio cholerae ), whereas others first encounter the host at a mucosal surface before they invade systemically (e.g., influenza virus). A distinctive immunoglobulin, secretory IgA, is produced at mucosal surfaces and is adapted to resist degradation and to function at these sites. Vaccines may be specifically designed to induce secretory IgA and thereby to block the essential initial steps in disease pathogenesis that occur on mucosal surfaces. Given its complexity, mucosal immunology has become a separate branch of the field of immunology.
Measurement of the Immune Response Immune response to vaccines are often gauged by the concentration of specific antibody in serum. Although seroconversion (i.e., transition from antibody-negative to antibody-positive status) serves as a dependable indicator of an immune response, it does not necessarily correlate with protection unless serum antibody is the critical mechanism in vivo and the levels achieved are sufficient (e.g., against measles). In some instances, serum antibody correlates with clinical protection but does not directly mediate it (e.g., vibriocidal serum antibodies in cholera).
Herd Immunity Successful vaccination protects immunized individuals from infection, thereby decreasing the percentage of susceptible persons within a population and reducing the possibility of infection transmission to others. At a definable prevalence of immunity, an infectious organism can no longer circulate freely among the remaining susceptibles . This indirect protection of unvaccinated (nonimmune) persons is called the herd immunity effect; through this effect, vaccination programs may confer societal benefits that exceed individual costs. The level of vaccine coverage needed to elicit herd immunity depends on the patterns of interaction among individuals within the population and the biology of the specific infectious agent. For, example, measles virus and VZV have high transmission rates and require a higher level of vaccine coverage for herd immunity than do organisms with lower transmission rates, such as S. pneumoniae. Wherever herd immunity for poliomyelitis and measles has been induced with vaccines, transmission of infection has ceased; however, herd immunity may wane if immunization programs are interrupted (as was the case for diphtheria in the former Soviet Union) or if a sufficient percentage of individuals refuse to be immunized because of a fear of vaccine-related adverse events (as occurred for pertussis in the United Kingdom and Japan). In either setting, the loss of herd immunity has led to renewed circulation of the organism and subsequent large outbreaks with serious consequences.
PRINCIPLES OF VACCINE USE


Route of Administration Microbes differ in their routes of infection, patterns of transmission, and predispositions for certain age groups. The route of vaccine administration (oral, intranasal, intradermal, transdermal, subcutaneous, or intramuscular) takes these factors into account in order to maximize protection and minimize adverse events. Vaccine development is more a pragmatic undertaking than an exact science, guided only in party by immunologic principles and shaped largely by the results of clinical trials. While vaccines can theoretically be given by any route, each vaccine has unique characteristics adapted to a particular route and, in practice, must be given by the licensed route, for which optimal immunogenicity and safety have been documented. For example, vaccines containing adjuvants are designed for injection into the muscle mass. Mucosal administration of vaccines designed for parental administration may not induce good systemic responses because such vaccines do not induce mucosal secretary IgA. Administration of hepatitis B vaccine into the gluteal rather than the deltoid muscle may fail to induce an adequate immune response, while SC rather than IM administration of DTaP vaccine increases the risk of adverse reactions. Injectable biologicals should be administered at sites where the likelihood of local, neural, vascular, or tissue injury is minimized.
Age Because age influences the response to vaccines, schedules for immunization are based on age-dependent responses determined empirically in clinical trials. The presence of high levels of maternal antibody and/or the immaturity of the immune response to some vaccines (e.g., measles and pneumococcal polysaccharide vaccines) but not to others (e.g., hepatitis B vaccine). In the elderly, vaccine responses may be diminished because of the natural waning of the immune system, and larger amounts of an antigen may be required to produce the desired response (e.g., in vaccination against influenza). In contrast, in some age groups, the use of substandard amounts of antigen is sufficient for immunity induction and reduces the risk of adverse effects (e.g., a reduced dose of diphtheria toxoid for persons≥7 years of age). Age-related adverse events are discussed in a later section.
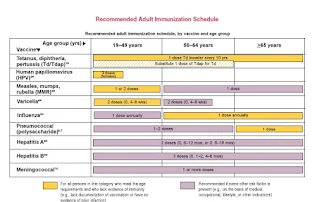
 Target Populations and Timing of Administration Disease attack rates differ across the human life span, and the timing of immunization must consider these variations along with age-specific response to vaccines, the durability of the immune response, and the logistics for optimal identification and vaccination of the groups at risk. Aside from immunologic parameters, many factors are involved, including demographic features; thus, vaccination programs are really as much community as individual endeavors. Schedules for immunization are ultimately derived from careful consideration of the many relevant variables and may ultimately depend on the best opportunities to reach the target groups (e.g., infancy, school entry, puberty, college enrollment, military induction, entry into the workplace). Health care workers administering vaccines or caring for patients with vaccine-preventable diseases have a special responsibility to be adequately immunized themselves and to take all necessary precautions to minimize the risk of spreading infection (e.g., hand washing between immunizations or other interactions with patients). Catch-up immunization schedules for infants and children through the age of 18 years have been approved by the CDC. For common and highly communicable childhood diseases such as measles, the target population is the universe of susceptible individuals, and the time to immunize is as early in life as is feasible and effective. In the industrialized world, immunization with live-virus vaccine at 12-15 months of age has become the norm because the vaccine protects >95% of children immunized at this age and there is little measles morbidity or mortality among infants < 1 year of age. In contrast, under crowded conditions in the developing world, measles remains a significant cause of death among young infants. For optimal benefit in this situation, it is necessary to immunize early enough to narrow the window of vulnerability between the rapid decline of maternal anti-body 4-6 months after birth and the development of vaccine-induced active immunity; this choice must be made despite the less efficient immune response in children < 1 year old.
Target Populations and Timing of Administration Disease attack rates differ across the human life span, and the timing of immunization must consider these variations along with age-specific response to vaccines, the durability of the immune response, and the logistics for optimal identification and vaccination of the groups at risk. Aside from immunologic parameters, many factors are involved, including demographic features; thus, vaccination programs are really as much community as individual endeavors. Schedules for immunization are ultimately derived from careful consideration of the many relevant variables and may ultimately depend on the best opportunities to reach the target groups (e.g., infancy, school entry, puberty, college enrollment, military induction, entry into the workplace). Health care workers administering vaccines or caring for patients with vaccine-preventable diseases have a special responsibility to be adequately immunized themselves and to take all necessary precautions to minimize the risk of spreading infection (e.g., hand washing between immunizations or other interactions with patients). Catch-up immunization schedules for infants and children through the age of 18 years have been approved by the CDC. For common and highly communicable childhood diseases such as measles, the target population is the universe of susceptible individuals, and the time to immunize is as early in life as is feasible and effective. In the industrialized world, immunization with live-virus vaccine at 12-15 months of age has become the norm because the vaccine protects >95% of children immunized at this age and there is little measles morbidity or mortality among infants < 1 year of age. In contrast, under crowded conditions in the developing world, measles remains a significant cause of death among young infants. For optimal benefit in this situation, it is necessary to immunize early enough to narrow the window of vulnerability between the rapid decline of maternal anti-body 4-6 months after birth and the development of vaccine-induced active immunity; this choice must be made despite the less efficient immune response in children < 1 year old.
Invasive infections due to Hib (meningitis, pneumonia, and epiglottitis) occur primarily in young children, with rates rising sharply after the disappearance of maternally derived antibody. First-generation Hib polysaccharide vaccines often failed when administered during infancy because very young children cannot respond to pure polysaccharides. This problem has been overcome by conjugating the capsular polysaccharide with a protein to create a T cell-dependent antigen, to which infants effectively respond.
 In contrast, rubella is primarily a threat to the fetus rather than to infants and young children. The ideal strategy would be to immunize all women of reproductive age before they became pregnant. Because it is difficult to ensure this type of coverage, rubella is included in a combination vaccine with measles and mumps (MMR) that is administered during infancy and boosted at the age of 4-6 years. It is recommended that pregnant women be screened for rubella antibodies and that seronegative women be given rubella vaccine after delivery. Similar considerations apply to the use of the vaccine against HPV that was recently approved in the United States and is intended primarily to prevent cervical cancer in women. Accordingly, it is recommended that the vaccine be given at the age of 11-12 years (or as early as 9 years), so that all are immunized before becoming sexually active.
In contrast, rubella is primarily a threat to the fetus rather than to infants and young children. The ideal strategy would be to immunize all women of reproductive age before they became pregnant. Because it is difficult to ensure this type of coverage, rubella is included in a combination vaccine with measles and mumps (MMR) that is administered during infancy and boosted at the age of 4-6 years. It is recommended that pregnant women be screened for rubella antibodies and that seronegative women be given rubella vaccine after delivery. Similar considerations apply to the use of the vaccine against HPV that was recently approved in the United States and is intended primarily to prevent cervical cancer in women. Accordingly, it is recommended that the vaccine be given at the age of 11-12 years (or as early as 9 years), so that all are immunized before becoming sexually active. Some vaccines, such as the influenza and polyvalent pneumococcal polysaccharide products, were originally formulated to prevent pneumonia hospitalizations and deaths among the elderly. These products have been consistently underused, in large part because physicians and otherwise-healthy older individuals ignore the recommendations but also because vaccines continue to be thought of as interventions for infants and children. There is considerable debate about alternative strategies to reduce the burden of these diseases in the elderly by indirectly protecting them through childhood vaccination, which would reduce transmission. The development of new vaccines and the exploitation of new routes of administration may facilitate this approach; examples include the development of pneumococcal conjugate vaccines and the administration of influenza vaccine by the intranasal route, respectively. The pneumococcal conjugate vaccine has made it possible to immunize young infants at risk of pneumococcal pneumonia, meningitis, and otitis media, but whether immunity will persist or will need boosting in adulthood remains to be determined. What is clear is that the number of recommended vaccines and the strategies for their deployment are undergoing constant revision.
Adjuvants The immune response to some antigens is enhanced by the addition of adjuvants-nonspecific boosters of immune responses. Adjuvants include aluminum salts or, in the case of polysaccharides such as the polyribose phosphate oligosaccharide of Hib, a carrier protein to which the polysaccharide is conjugated. Adjuvants are essential to the efficacy of a number of inactivated vaccines, including diphtheria and tetanus toxoids, acellular pertussis vaccine, and hepatitis B vaccine; they also appear to be required for enhancement of the response to killed H5N1 avian influenza vaccines. The mechanism by which adjuvants enhance immunogenicity is not well defined but appears to relate to the ability of the adjuvant to activate antigen-presenting cells, frequently through stimulation of Toll-like receptors. Other reported mechanisms for adjuvant effects include rendering of soluble antigens into a particulate form, the slowing down of antigen release in order to prolong stimulation of the immune response. Identification of new adjuvants that are safe, more effective, and inexpensive is a high priority for vaccine researchers and manufacturers.










No comments:
Post a Comment